Maximize clinical trial success with model-informed precision dosing
Empower Biopharmaceutical companies to incorporate precision dosing into clinical trials with an integrated and quality-compliant precision dosing platform.

Increase Drug Program Success with Precision Dosing Intelligence.
Plan and execute drug optimization strategies starting in Phase I.
Leverage precision dosing intelligence (a combination of MIPD, Bayesian forecasting, and machine learning) to test multiple dose ranges and identify targeted dose level and interval.
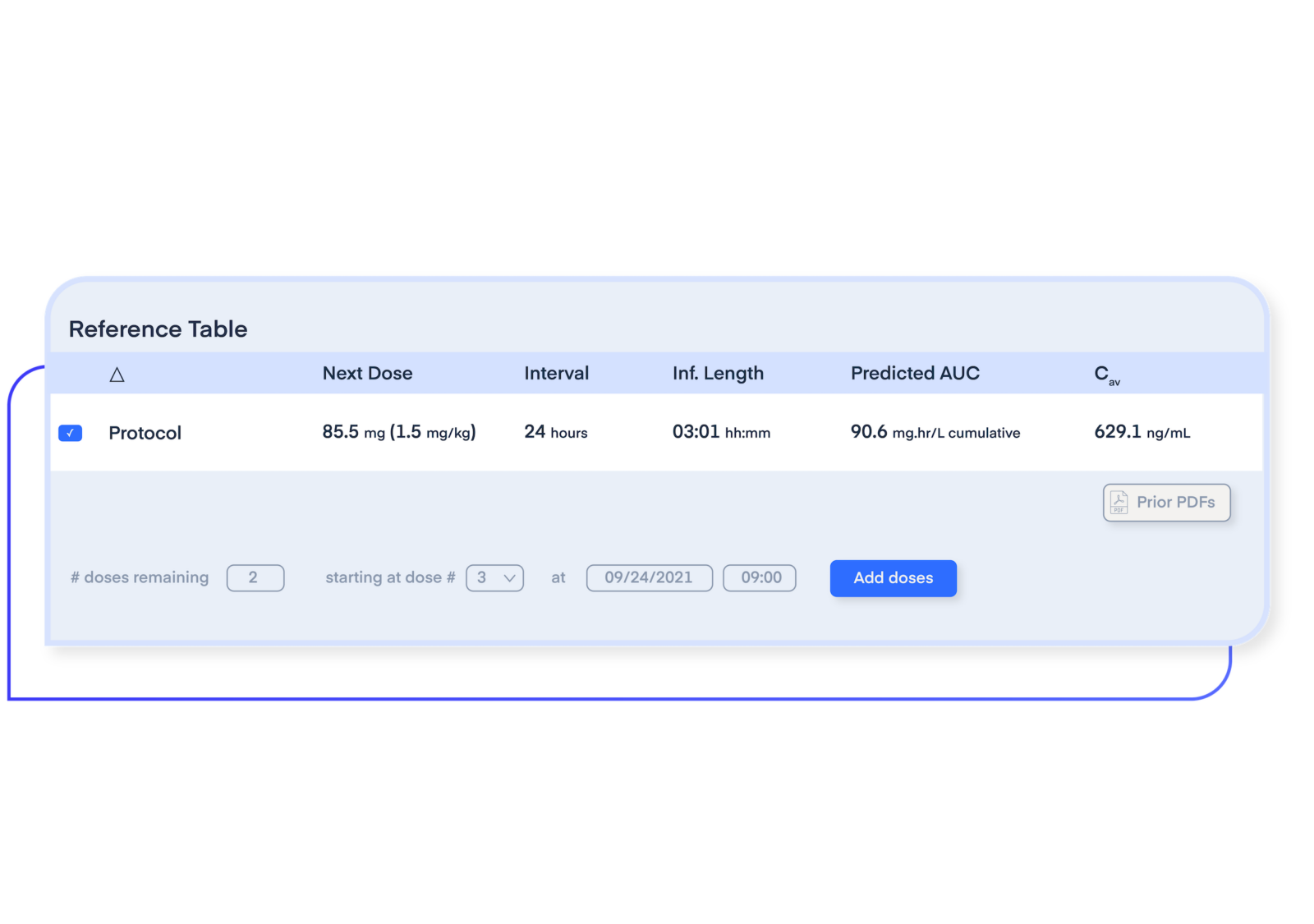
Maximize drug efficacy and safety for patients.
Through a combination of drug monitoring and precision dosing, subtherapeutic and supratherapeutic doses can be avoided by targeting a desired drug exposure or biomarker range by taking into account concentration levels, lab results, and demographic data from an individual patient.
Avoid the pitfalls of designing a dose regimen based on maximum therapeutic dose (MTD), which can give rise to many downstream issues, including adverse events.
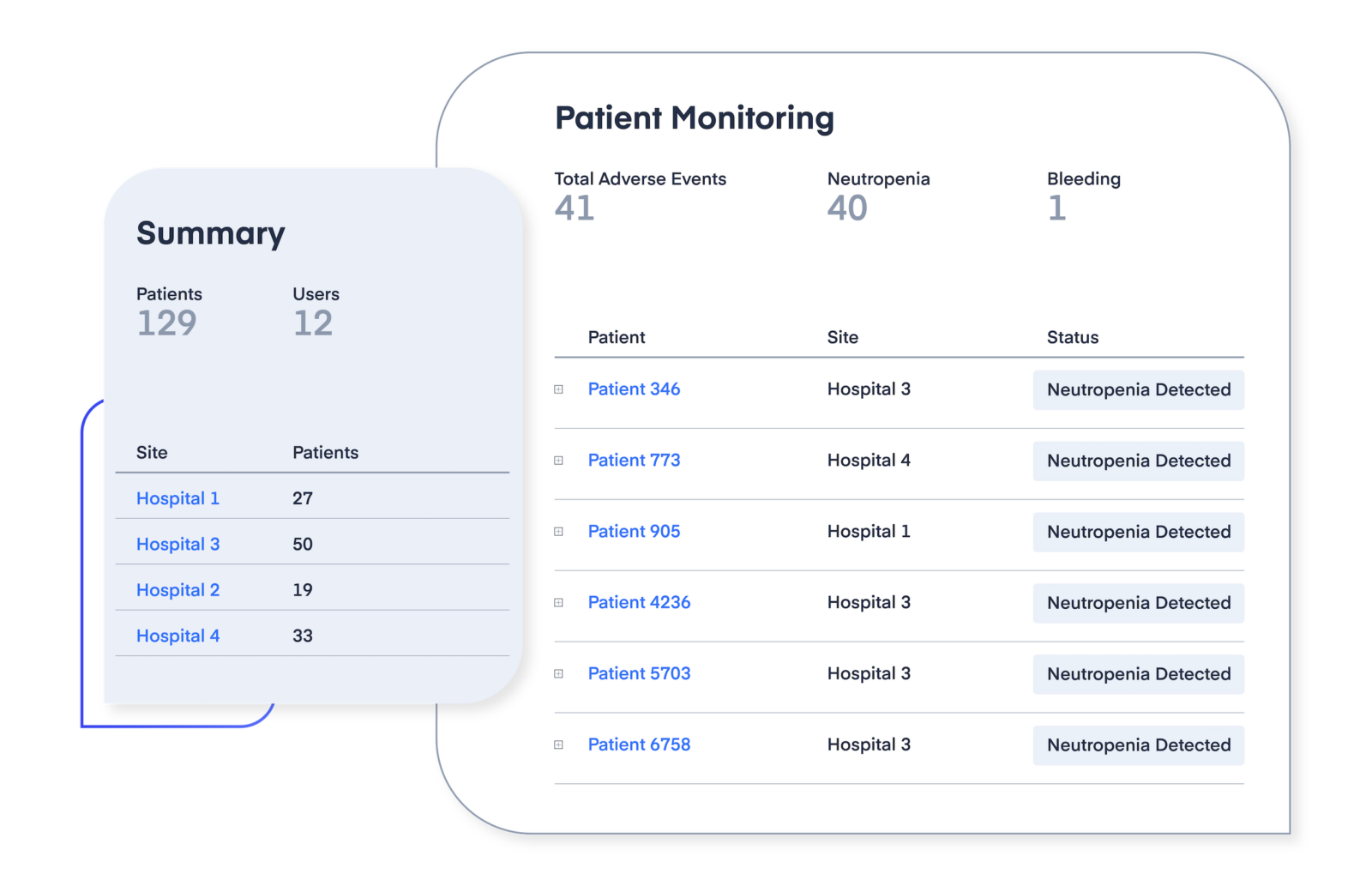
Maximize patient enrollment and retention in clinical trials.
High inter-patient variability may make it challenging to reduce the risk of an adverse drug reaction for patients enrolled in a clinical trial. High rates of adverse drug events may lead to lower patient retention during the clinical trial. Approximately 30% of patients drop out of clinical trials, resulting in heavy financial costs. Precision dosing can help achieve therapeutic targets and reduce the chances of adverse drug event-related outcomes.
Real-time drug response predictions can help identify vulnerable patients prior to or early on in treatment, stemming the risk of dropout and increasing the likelihood of successful therapy.
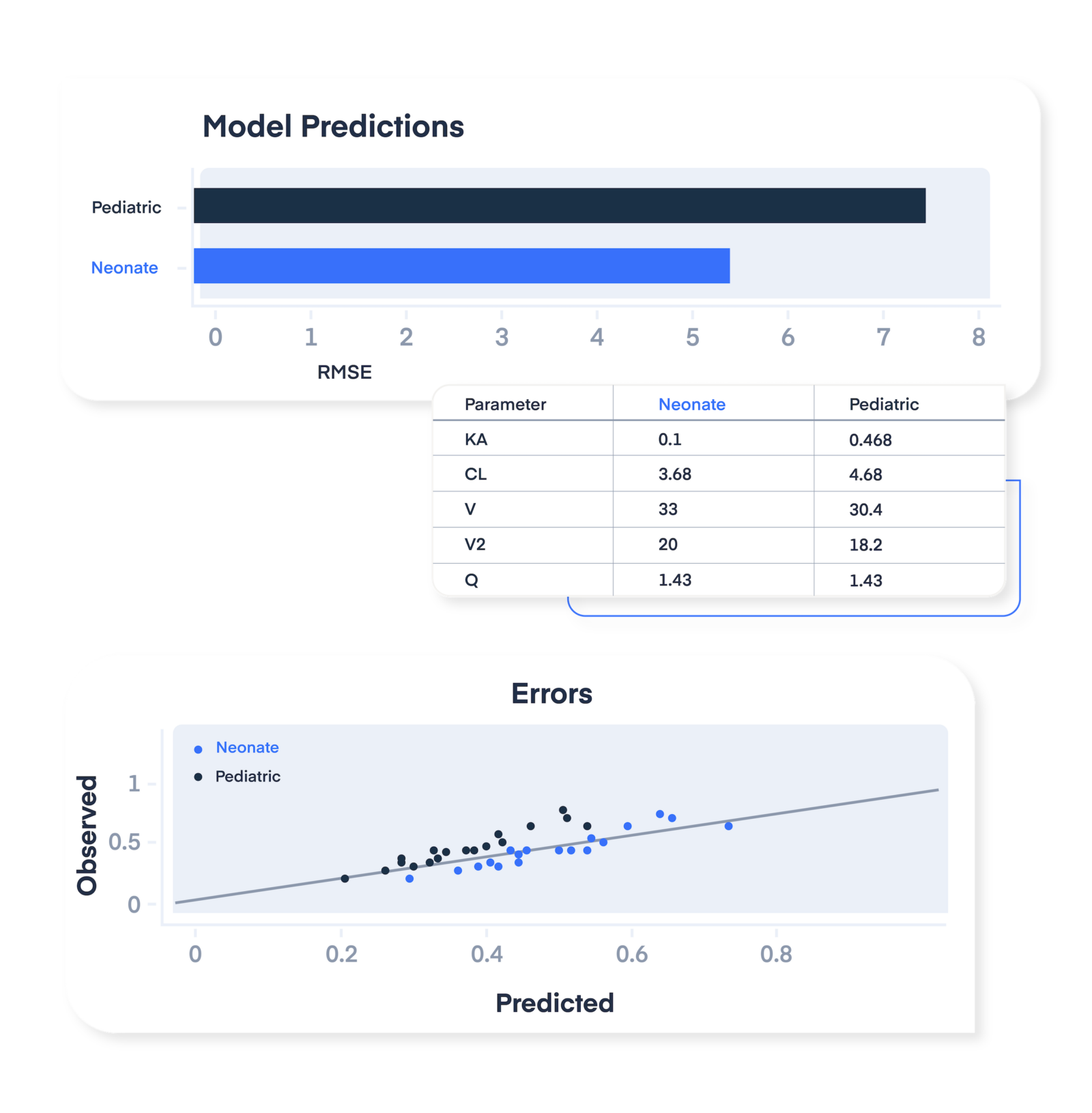
Enable label expansion into new indications.
Prove drug efficacy and safety for patient populations beyond the original use for which the drug was designed through concentration or biomarker-guided model-informed precision dosing support.
Provide a streamlined path for label updates incorporating RWD to update dosing guidance or seek approval in alternate treatment groups.